Informed Choice News
The 4 Steps to Informed Choice Certification

Supplement users are increasingly seeking transparency in the products they purchase with 71% of consumers looking for a quality assurance mark when shopping for dietary supplements. With rising inflation and fierce competition within the nutrition industry, supplement companies are using Informed Choice dietary supplement testing to set themselves apart.
Informed Choice is a global quality assurance program developed to minimize the risk of dietary supplements being inadvertently contaminated with banned and harmful substances. Our 130+ supplement brands utilize certification to display their dedication to quality assurance and to provide confidence for their customers.
Products tested and certified by Informed Choice undergo a rigorous four-step certification process.
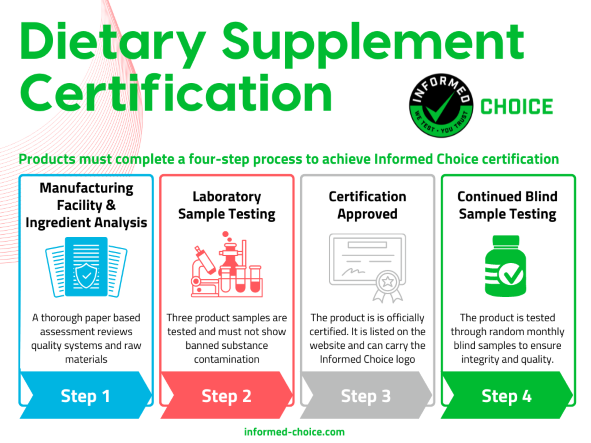
|
Step 1: Manufacturing Facility and Ingredient Analysis
Prior to supplement certification, Informed Choice analyzes the ingredient list of the product, along with a paper-based manufacturing assessment to ensure the facility is following proper quality assurance procedures and is not handling banned substances. A manufacturing facility must meet program requirements and successfully complete the Manufacturing Assessment Questionnaire or the product will be eligible for certification.
The manufacturing assessment includes a thorough review of the following:
- Quality systems and audits
- Staff training in relation to cross-contamination prevention
- Raw materials
- Raw material supplier assessment procedures
- Traceability & recall procedures
Step 2: Sample Testing
Included in the pre-certification process is sample testing. Our ISO 17025 accredited testing labs in the US or UK must test three samples across at least three production runs/batches. These samples must not show an indication of contamination with prohibited substances.
Step 3: Approved Certification
If no banned ingredients are included in the raw material list, the manufacturing facility passes the assessment and the tested samples do not show evidence of contamination, the product will be awarded certification with Informed Choice. The dietary supplement brand will then be allowed to use the Informed Choice logo to market the product in compliance with the Informed Choice brand standards. This document showcases the best marketing practices to leverage the new certification.
The newly certified product will also be listed on the Informed Choice website. This includes a dedicated product page that showcases product goals, formulation, regional availability, and links to purchase. All tested batch numbers will also be listed.
Step 4: Post-Certification Testing
Following supplement certification, each product must undergo at least monthly blind sample testing to independently ensure the integrity of the product. Blind samples are purchased by the Informed Choice Program Management team from retail outlets.
Batches that have been tested for the product are then published on the Informed Choice website. In addition to monthly product testing, the product and manufacturing facilities are regularly re-reviewed to ensure the requirements of Informed Choice continue to be met.










