Informed Choice News
Clean Living Starts with an Informed Choice: Key Elements of the Informed Choice Program

August 27, 2021
Results from a 2020 survey by the Council of Responsible Nutrition (CRN), showed that 91% of consumers who already used supplements have increased their usage since the onset of the COVID-19 pandemic. The top reasons for this increase were cited as immune support and health/wellness benefits. But, could the supplements being purchased to provide health benefits actually be contaminated or hiding banned or harmful substances undeclared on the label?
Based on LGC’s research and retail surveys, as many as one in ten supplements on the market could be contaminated with unsafe or banned substances. Although the contamination levels may be at a low concentration, the levels could still be enough to pose health risks to those who ingest them. With the ever-growing supplement use worldwide, it is vital for consumers to choose dietary supplements that have been third-party tested to minimize these risks.
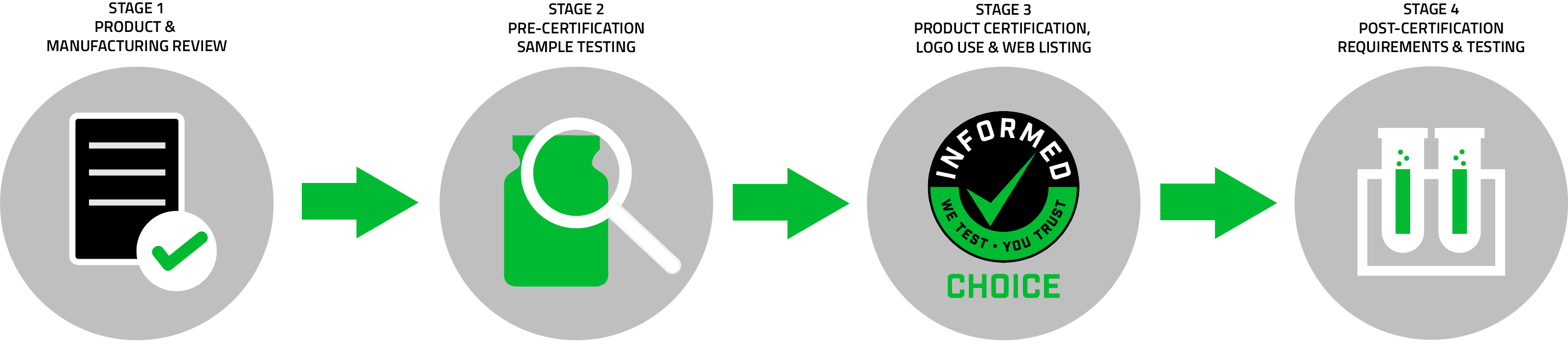
Developed in 2007, Informed Choice is a global quality assurance certification program for the dietary supplement industry. Recognized by numerous anti-doping and nutrition bodies around the world, the program helps consumers make safer and more reliable choices when looking for dietary and sports supplements. When a consumer sees the Informed Choice logo on the product label, they can feel confident knowing that it has been regularly tested for more than 285 prohibited and potentially harmful substances and was manufactured under high-quality standards. While there are several important aspects of the program, three of the key elements of the Informed Choice program include:
-
Stringent Manufacturing Assessment
-
Accredited Testing Methods
-
Retail Monitoring
Manufacturing Assessment
Informed Choice performs an extensive pre-certification assessment by way of a manufacturing assessment questionnaire. A brand and manufacturer must provide documents to allow a detailed audit of every ingredient in the facility producing the product, formulation, and manufacturing cleaning procedures in order to meet the requirements for certification and examine potential banned substance contamination risks.
While Good Manufacturing Practice (GMP) is important, it is a common misconception to believe that GMP certification adequately addresses the risks of banned substances entering the manufacturing process of a supplement. Evidence of contamination in GMP facilities is not uncommon because the handling of steroids, stimulants, SARMS and other substances banned by the World Anti-Doping Agency (WADA) is permitted under GMP regulations. Informed Choice requires manufacturers producing certified products to go beyond GMP to minimize risk.
|
Additionally, a raw material inventory list must be provided which details how raw materials are handled and stored within a manufacturing facility. This allows Informed Choice’s team of experienced assessors to thoroughly check for any compounds banned or deemed high-risk for cross-contamination. Should any corrective actions be recommended, a product will not be awarded certification until the actions are completed and approved.
ISO 17025 Accredited Testing
ISO/IEC 17025 accreditation is an international laboratory and testing standard. Any tests that are accredited to this standard have been developed and validated in line with international requirements and are independently assessed/evaluated objectively by third-party industry experts. All Informed Choice certified supplement brands are tested using ISO/IEC 17025 accredited methods. Within the UK (LGC Fordham), accreditation is granted by UKAS (the United Kingdom Accreditation Service - laboratory number 1187). Within the US (LGC Lexington), accreditation is granted by A2LA (the American Association for Laboratory Accreditation - certificate no. 3244.01).
The Informed Choice supplement screen is backed by LGC and has over 65 years of anti-doping experience. Currently, over 25,000 samples are tested annually for 285+ substances banned in sports. These substances include the drugs of abuse, anabolic agents, stimulants, beta-2-agonists, masking agents, diuretics and new and emerging threats such as SARM's, vaptans and PPAR's, etc.
Retail Monitoring
Once a sports nutrition supplement is tested and certified by Informed Choice, it will then undergo at least monthly blind sample testing to ensure the integrity of the product. This means a sample of the certified product is purchased at random from online and physical retail outlets by the Informed Choice program management team. It ensures the supplements being tested are the same as the supplements consumers are able to purchase in stores or online.
Supplement consumers must ensure that the products they use are tested by a third-party supplement testing company to minimize the risks of ingesting a banned substance or compound. Informed Choice does not perform composite testing, a practice where multiple lots or batches can be combined into one sample and then tested. Such a process introduces an unnecessary risk since the possibility exists that a contaminated batch may not be detected due to dilution with other samples from clean production batches.
Bulk testing is also a common practice, where one large batch of product is produced, tested and certified before it has been packaged. Manufacturers will then package portions of the bulk batches on different dates, depending on inventory requirements. In such cases, opened bulk material may be processed on separate packaging lines on different dates, creating an opportunity for cross-contamination after the bulk batch is tested and certified.
Upon passing the banned substance screen, all batch numbers for tested products are listed on each individual product page on informed-choice.com

|
Quality assurance for the long run
Over 130 brands worldwide have chosen Informed Choice certification to take their quality assurance practices to the next level. With the majority of consumers looking for a quality mark or seal when purchasing supplement products, providing added quality assurance through third-party certification is more valuable than ever.










